At the start of the COVID-19 pandemic, Health Canada issued an interim policy to allow the import of products that do not fully meet regulatory requirements, but were comparable, to meet the incredibly high demand for disinfectants and hand sanitizers.
This policy allowed the government to resolve 25 Tier 3 drug shortages and 118 medical device shortages. In fact, as of February 17, 2021 a total of 56 drugs and 265 medical devices were permitted to be imported under this measure.
Yesterday, on March 1, 2021, Health Canada replaced this policy with a new, second Interim Order for COVID-19 drugs, medical devices, and specific foods. The intent is to alleviate the potential for shortages that may arise from COVID-19, either directly or indirectly.
So, what’s changed?
Here’s a comparison on the key regulatory differences between Interim Order No. 1 and No. 2.
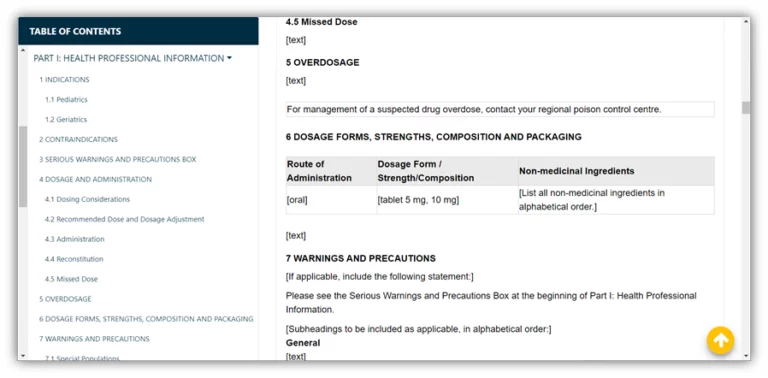
| Provision | Interim Order No. 1 | Interim Order No. 2 |
| List of drugs and medical devices eligible for exceptional import | Yes | Yes |
| Regulatory framework for exceptional import and sale of surface disinfectants (biocides) | Yes – biocides refer to disinfectants and hand sanitizers | Yes – biocides refer to disinfectants only, NOT hand sanitizers |
| Requirement for DEL to conduct activities related to drug-based hand sanitizers | DEL is NOT required | DEL is required |
| Drug-based hand sanitizer labels | Mock-up labels are NOT required, text is acceptable | Mock-up labels are required |
| Requirements for official language (English and French) for exceptional importation | Yes | Yes – explicitly required |
The Health Canada deadline for many of the above change in requirements, for example DEL application submission, is 6 months.
For more information and support in preparing the necessary regulatory applications under the new Interim Order for COVID-19 products, please contact us at info@axsource.com.