
What is KASA?
KASA is an assessment tool that was designed to maximise the efficiency of the regulatory review process. The tool relies on structured data to eliminate some of the redundancies of more traditional assessment formats (narrative text and lengthy summaries). KASA stands for Knowledge-Aided Assessment and Structure Application (KASA) and is part of FDA’s broader initiative to modernize regulatory assessment.
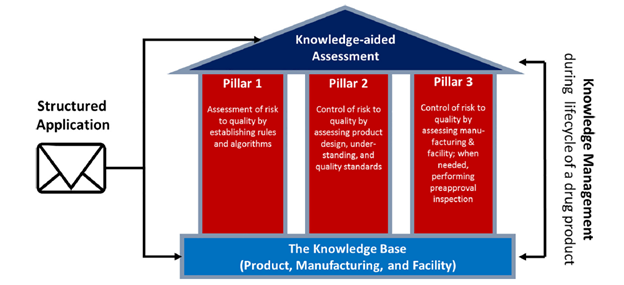
Fig. 1. Knowledge-aided Assessment & Structured Application (KASA) system.
KASA captures information on risk and controls for drug products, manufacturing, and facilities. This knowledge base is the foundation of KASA and includes historical information at FDA. Each pillar represents significant development phases for the KASA system: rules and algorithms to estimate product and manufacturing risk, risk control through assessment of product design, and risk control through assessment of manufacturing and facility.
Background, benefits & current use
KASA was developed by a group of OPQ reviewers in 2016, for solid dosage forms drug product. The idea was to address the following quality assessment challenges:

Currently, KASA is used in quality assessments for drug product, biopharmaceutics, manufacturing, and most recently, for drug substance. In scope applications include NDAs, ANDAs, BLAs and DMFs. Once submitted, applications are checked against others in the KASA database to identify any outliers in control strategy or risk.
KASA is hosted on CDER’s IT platform. Unstructured data from eCTD submissions is first extracted, then manually entered in FDA’s KASA system. The result is a structured set of data with standard formatting and a common vocabulary. This is what allows FDA Quality Assessors to easily search and retrieve desired information.
KASA for Drug Substance
For KASA Drug Substance, the following features have been developed:
1. Structured Drug Substance Synthetic Pathway (most complex)
Captures the assessment of synthetic steps. Based on data mining process followed by Quality Assessors. Includes control of starting materials, intermediates, and reagents.
2. Chemical Structures Library
Captures synthetic inputs/outputs and impurities from synthetic pathway steps.
3. Enhanced Drug Substance Risk Management
Assigns risk levels of high, medium, or low automatically. Based on pre-defined questions and associated FMECA scores for critical quality attributes (CQAs).
4. Drug Substance Analytics
Helps identify outliers in FDA database of applications. Based on data for each Drug Substance synthetic pathway.
Looking ahead
KASA was designed to capture and manage data more efficiently during the lifecycle of a drug product. To make the tool even more useful, the vision is to integrate KASA with structured applications. This would help automate the review process and reduce regulatory burden. The result? Accelerated regulatory decision-making, faster review times, and more accessible medicines.
For more information on preparing regulatory applications for FDA, contact AXSource today at info@axsource.com.
References:
- Lawrence, X. Yu, et al. “FDA’s new pharmaceutical quality initiative: Knowledge-aided assessment & structured applications.” International journal of pharmaceutics: X1 (2019): 100010.
- Wu, L. “Modernizing Drug Substance Assessment through KASA.” FDA SBIA API-Workshop (2021).


